Ensuring Quality
Welcome to the Certification Body of WMDA
Why gain WMDA Certification?
- Quality improvement: the processes within your organisation will be reviewed by external experts who are experienced in registry operations and in providing blood stem cell products internationally.
- World market acceptance: national health authorities may adopt WMDA as an assurance of the quality of imported blood stem cells. Blood stem cell products from another country may comply with national requirements when originating from a WMDA certified registry or cord blood bank.
- Visibility in the global Search & Match Service: when physicians select a donor, donors from WMDA Benchmark L1/L2 and Full Standards compliant registries are marked with the certification logo. This assures physicians that the donor is listed in an organisation that promotes the quality of procedures necessary to obtain, in the shortest possible time, the appropriate quality and quantity of blood stem cells.
WMDA Standards
- processing incoming requests for access to donors/cord blood units arriving from organisations in other countries;
- facilitating outgoing requests for international donors/cord blood units for patients in the country where the registry resides;
- coordinating the activities of donor, collection and transplant centres and cord blood banks within a country.
Organisations (registries) demonstrate their commitment to comply with WMDA Standards.
Quick references:
- 2020 WMDA Standards
- Guidance (request access by sending an e-mail to mail@wmda.info)
- 2024 WMDA Standards (effective July 1, 2024)
Two steps to become WMDA Certified
To become WMDA Benchmark L1/L2 certified, an organisation must meet all benchmarked WMDA Standards. The review will focus on how the registry has organised its processes. Trained volunteers review the electronic submission of written documents covering the registry’s policies and procedures. Benchmark L1 is awarded to registries with low activity and can be renewed; Benchmark L2 is awarded to registries meeting a minimum activity of six donations in the last three years with two being international. The next step after Benchmark L2 is Full Standards certification. After receiving its Benchmark L1 or L2 certificate, an organisation is required to perform a mid-cycle surveillance in the second year of a four year cycle.
The second step is to become compliant with all required WMDA Standards:
Only WMDA Benchmark L2 registries can apply for WMDA Full Standards certification. To become WMDA Full Standards compliant, an organisation must meet all required WMDA Standards that cover all steps between donor recruitment and donor follow-up after blood stem cell donation. After receiving its Full Standards certificate, an organisation is required to perform a mid-cycle surveillance in the second year of a four year cycle. Once WMDA Full Standards compliant, a registry must apply for re-certification every four years.
Organisation of the certification programme
- WMDA Standards, the basis of evaluation
- Requirements for submitting an application (Policy: Certification Body Application Requirements And Levels)
- Stepwise progress towards certification (Policy: Certification Body Application Requirements And Levels)
- Certification agreement listing rights and duties of applicants
- Evaluation process
- Procedures for handling complaints and appeals
- Fees
- Sources of funding
Become a reviewer
- you will help organisations to give patients the best possible care;
- you will become an expert on WMDA Standards;
- you will learn new practices and operations from other organisations;
- you can share knowledge and expertise with others;
- you will become part of a network of colleagues;
- you will discover a variety of ways to meet WMDA Standards;
- you will be able to reflect on your own organisation’s practices;
- you will travel internationally for onsite audits; and
- you will learn how to use online tools and resources created for reviewers.
Applicants who wish to become a reviewer must meet certain criteria and be approved by the Accreditation Steering Committee. Once you are accepted as a reviewer, you will have to complete the online training programme within six months. Next, you will be required to participate as a trainee in a document review for a WMDA Benchmark L1/L2 or Full Standards application. The final step will be for you to perform an onsite (or remote) audit with an experienced reviewer.
After becoming a trained reviewer, you will be asked to assist in one to two reviews a year and will also have to complete continuing education. Interested? Contact WMDA office and ask for the details.
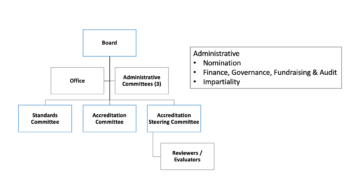
Governance Certification Body
Certification Body
The aims of the Certification Body are to:
- Promote harmony between worldwide stem cell donor registries and cord blood banks
- Encourage uniformity of practice based on WMDA Standards
- Administer the WMDA certification programme.
The main activities of the WMDA Certification Body are focused in three Committees: WMDA Standards Committee, WMDA Accreditation Committee, and WMDA Accreditation Steering Committee. These three committees report directly to the WMDA Board. Administrative Committees are responsible for Nominations, Finance and Impartiality of the Certification Body.
WMDA Certification Body
Partnerships with other accreditation bodies
Since 2000 the Foundation for the Accreditation of Cellular Therapy (FACT) has operated an accreditation programme for cord blood banks in collaboration with the NetCord Foundation. The FACT accreditation programme is an opportunity for cord blood banks to show that they comply with internationally accepted standards.
On 1 January 2017 the activities of NetCord Foundation were merged into WMDA. Since then, WMDA has worked with the FACT office to promote the accreditation programme among cord blood banks. The FACT website provides information about the application process for accreditation.